- ← Previous item
- Pos.-No. 23211541
- Next item →
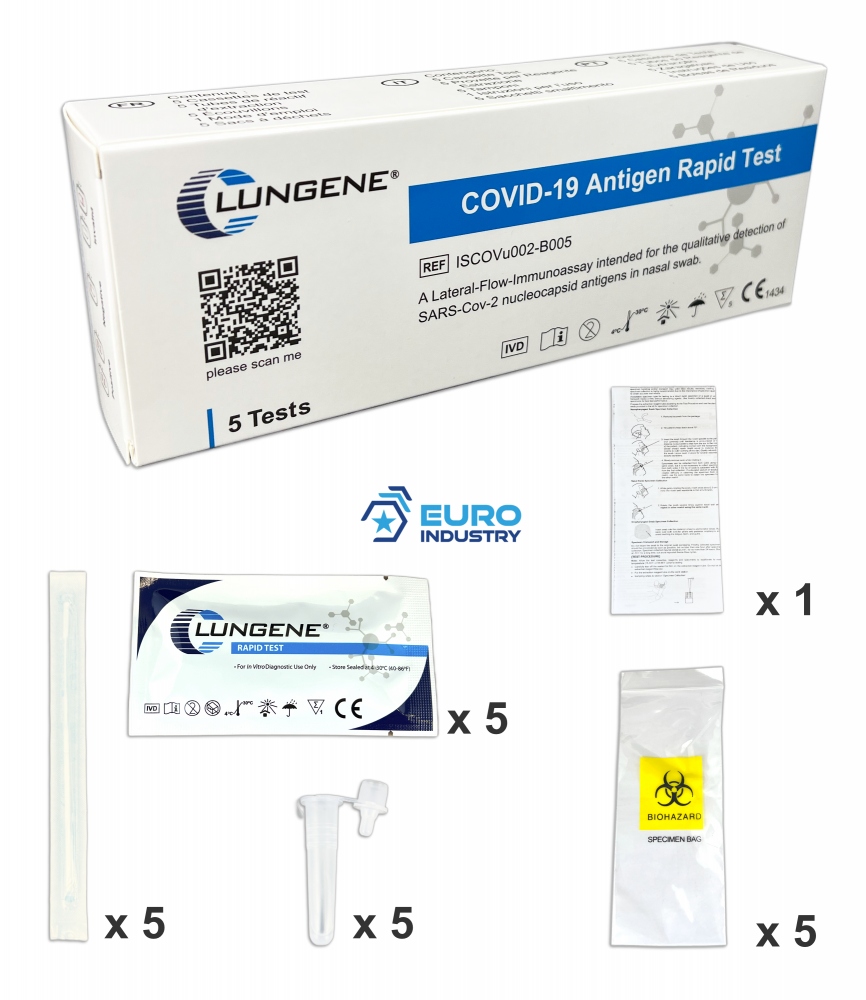
Clungene Profi nasal-swab COVID-19 Box with 5 antigen auto test
Clungene Profi nasal-swab COVID-19 Box with 5 antigen auto test
€ 0.06 0.06 incl. VAT (0.05 excl. VAT), per piece *
- 60%
€ 0.15 incl. VAT (0.13 excl. VAT)*
€ 0.15 incl. VAT (0.13 excl. VAT)*
Availability: In Stock - shipping immediately
Packaging unit: 1 box
Manufacturer: Clungene
Country of origin: CN/0720
Customs tariff number: 38221900
Weight per piece: 150 g
Please give the recipient email address, as well as your name and email address.
The product description does not help you?
No problem. Simply fill out the form and we will answer you as soon as possible.
No problem. Simply fill out the form and we will answer you as soon as possible.
Clungene COVID-19 Rapid Antigen Test for Home, Set of 5 Applications, Nasal Sampling
Expire date: 2024-01
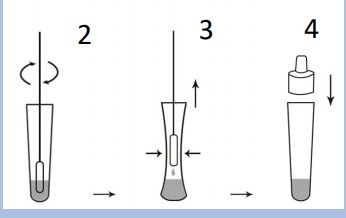
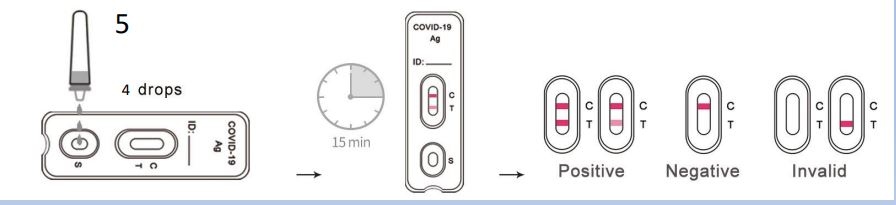
The COVID-19 Antigen Rapid Test is a lateral flow immunoassay based on the principle of double antibody sandwich technology. The SARS-CoV-2 nucleocapsid protein monoclonal antibody conjugated with colored microparticles is used as a detector and sprayed onto the conjugation pad. During the test, the SARS-CoV-2 antigen in the sample interacts with the SARS-CoV-2 antibody, which is conjugated with colored microparticles, creating an antigen-antibody-labeled complex. This complex migrates via capillary action on the membrane to the
test line, where it is captured by the pre-coated monoclonal SARS-CoV-2 nucleocapsid protein antibody. A colored test line (T) would be visible in the result window if SARS-CoV-2 antigens are present in the sample. The absence of the T-line indicates a negative result. The control line (C) is used as a procedural control and should always be displayed when the test procedure is being carried out properly. Until lay approval from the BfArM is available, the test is only approved for medical or trained personnel.
File number of the special approval of the BfArM 5640S 168/21
Expire date: 2024-01
The COVID-19 Antigen Rapid Test is a lateral flow immunoassay based on the principle of double antibody sandwich technology. The SARS-CoV-2 nucleocapsid protein monoclonal antibody conjugated with colored microparticles is used as a detector and sprayed onto the conjugation pad. During the test, the SARS-CoV-2 antigen in the sample interacts with the SARS-CoV-2 antibody, which is conjugated with colored microparticles, creating an antigen-antibody-labeled complex. This complex migrates via capillary action on the membrane to the
test line, where it is captured by the pre-coated monoclonal SARS-CoV-2 nucleocapsid protein antibody. A colored test line (T) would be visible in the result window if SARS-CoV-2 antigens are present in the sample. The absence of the T-line indicates a negative result. The control line (C) is used as a procedural control and should always be displayed when the test procedure is being carried out properly. Until lay approval from the BfArM is available, the test is only approved for medical or trained personnel.
File number of the special approval of the BfArM 5640S 168/21
- Sensitivity: 91.4% - 97.5%
- Specificity: 95.0% - 99.4%
- Expire date: 01-2024
- REF: ISCOVu002-B005
- 5 test cassettes
- 5 swabs
- 5 extraction tubes
- 5 buffer ampoules
- 1 instruction manual
No files available for download
| Property | GTIN/EAN | MPN (Manufacturer Part Number) |
|---|---|---|
| - | 6950921302728 | ISCOVu002-B005 |
* Price in Euro including VAT, plus forwarding charges.
In parentheses: price in Euro excluding VAT, plus forwarding charges.
Clients outside the EU as well as companies outside of Germany don't pay any VAT (excepting for shipping in Germany)!
 English
English  Français
Français Deutsch
Deutsch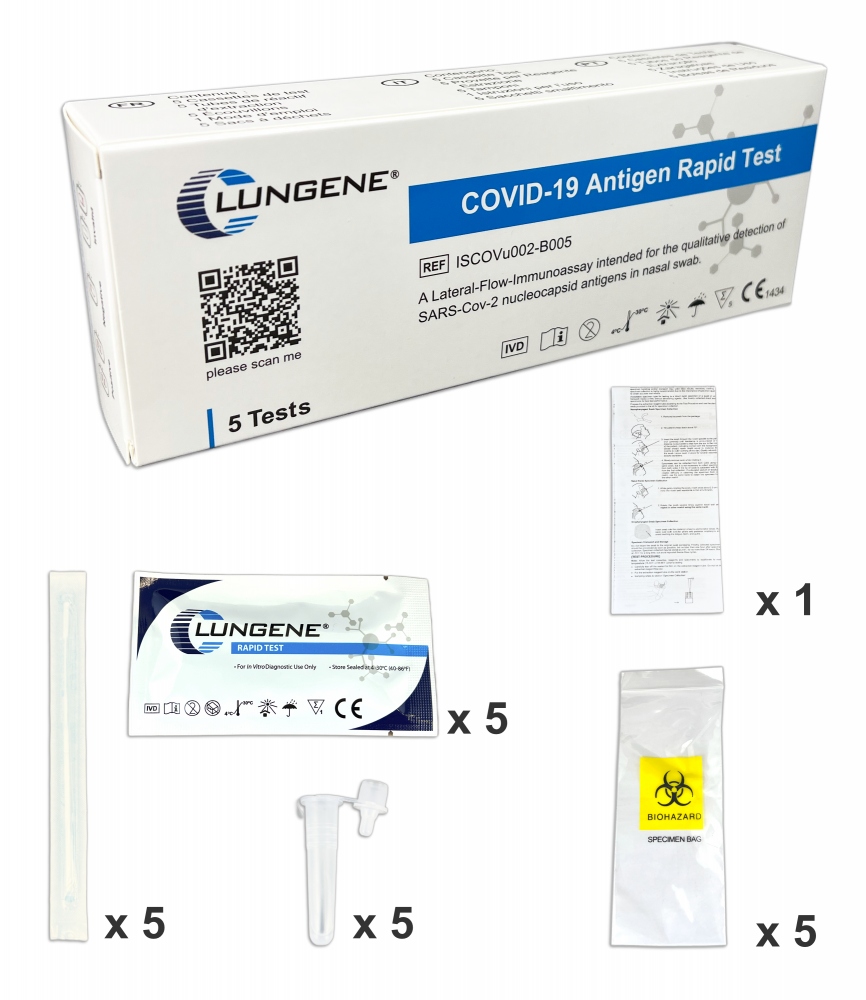


Follow us